WoW Woman in MedTech | Rachel Fallon, CTO at Sky Medical Technology
Interview by Marija Butkovic
Rachel brings nearly 30 years’ experience spanning Life Sciences and now the medical devices industry. From start-up through to FTSE100 companies (Amersham Biosciences, Procognia Ltd., Sense Proteomics Ltd. and Oxford Gene Technology), she has played a leading role in product R&D and operational functions, whilst contributing at executive level. Rachel has proven success in people development, change management, project management, R&D manufacturing and quality systems. As CTO of Sky Medical Technology, she has established the company's technical vision, product roadmaps and all aspects of cost effective technology development. Rachel leads R&D, Clinical Affairs, Quality & Regulatory Affairs, Manufacturing, Logistics and IT .
Rachel, tell us a bit about your background and your projects so far?
My interest and background has always been directed towards medical applications. I have worked in several life science companies from FTSE 50 to SME and a startup where I used my biochemistry and physiology degree as a base to bring molecular research tools to the market for academic research and drug discovery. A deep understanding of medical science together with the ability to appoint and work with multi-disciplinary teams building platform technologies including reagents, equipment and software has stood me in good stead for the last 10 years in medical devices.
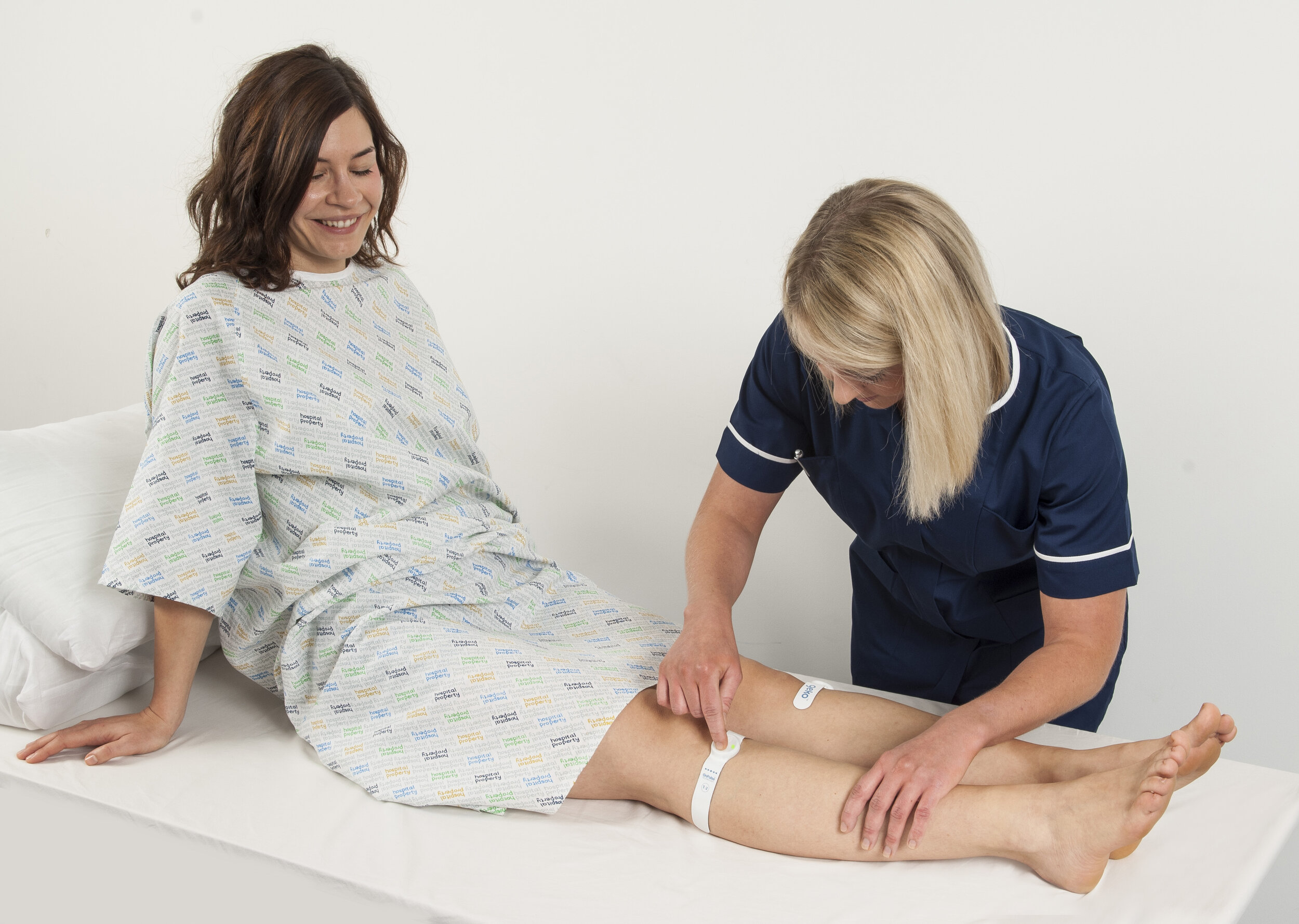
I am currently working to deliver a range of wearable medical devices from a platform technology capable of increasing blood circulation which is a valuable benefit for many patient groups including surgical patients, stroke patients and those with chronic wounds.
How did you get into this industry? Has it been an easy industry to get into or have you had many challenges?
Moving to a medical device business with a total of 6 employees in 2010 seemed like a natural progression for me coming from a life science businesses background; however, there were several new challenges. I went on a steep learning curve with respect to understanding medical device regulation. There was a requirement to design, develop and manufacture products in line with regulatory rules from the start. Working with knowledgeable consultants, our inventors (who had experience of clinical studies and biomechanical engineering) as well as external electrical engineering and industrial design consultants enabled me to form a product R&D team. We started building our clinical data based on case series and investigator led studies. The move to initiate Firstkind sponsored studies required me to appoint a team of clinical professionals. My wider experience and training in a large company setting stood me in good stead when building and managing multidisciplinary teams in all areas.
What does your current job role entail?
As CTO I work closely with the CCO to ensure that we develop and manufacture products and generate clinical data in line with regulations to support business propositions. I am responsible for the cross functional management of all operational aspects of the business including clinical and product R&D, Quality and Regulatory Assurance, Manufacturing and Logistics, and IT. I agree with plans, budgets and timelines with the heads of each function and ensure that the functions have the resources (human and budget) they need to deliver our business objectives. Developing and agreeing aligned strategies for each function serves to attract investors and motivate staff.
My wider team is full of creative, knowledgeable people and a particularly rewarding part of my job is to create an environment that encourages innovation through their innate creativity as well as ensuring that we are embracing new technologies and ideas from outside the company.
What projects are you working on at the moment?
We have a disposable wearable device for hospital use which addresses the risk of cross contamination by allocating one device to one patient and fitting a new device every 24 hours.
We are developing a reusable multi-use wearable device for the homecare market taking account of the requirement to incorporate additional environmentally friendly or recyclable materials where possible.
We are moving our manufacturing from manual to automated device assembly and packaging in a footprint that can be replicated worldwide
We have regulatory clearance to sell our devices in over 30 countries globally and are continuing to extend indications for use.
Having demonstrated the mechanism of action for our technology and generated data showing efficacy for several clinical conditions for example the treatment of oedema we are continuing to run a pipeline of Randomized controlled studies; this will enable additional regulatory clearances and provide the data for reimbursement, supporting more business propositions, primarily in hospital use and wound care.
How long did it take you to be where you are now?
My experience spans 40 years’ overall, with the last ten years spent in medical devices as CTO.
What was the biggest obstacle?
I’d say my biggest obstacle has been the slow clinical study recruitment rates which in turn have meant long timelines in producing clinical data in support of marketing messages. The difficulty here is that clinical professionals have to include clinical studies alongside treating their patients in their already busy schedule.
What are your biggest achievements to date?
Delivering innovative products to the market for clinical benefit feels like a real achievement. I have always wanted my legacy to be positive patient outcomes in medicine and I believe that my work in the last 10 years in particular has done and continues to do just that. I have built and am proud to work with competent expert teams throughout my career.
What does the #WomenInTech movement mean to you? What are the challenges of being a woman in wearable tech / STEM?
With 40 years’ experience in the Life Science industry, I’ve come to realise that supporting and inspiring women in STEM is paramount. The #WomenInTech movement is the perfect opportunity to motivate women to work in the industry. For me, the movement should aim to encourage an organic interest in STEM for women and proactively support gender diversity, so women can realise their full potential.
There is currently a significant under-representation of women in MedTech which is a cause for concern for organisations and professionals in the industry. A reduced representation of female perspectives in business can limit innovation and miss alternative ways of working. In healthcare, for example, gender equality in the workplace is important when facing challenges surrounding serious challenges such as chronic ulcers, blood clot prevention and post-surgical complications.
Although women represent half of the UK workforce, only 24% are in STEM1. According to a Government apprenticeship report from 2019, less than nine percent of STEM apprentices are female2. Even though women are generally outnumbered in Medtech, there is an equal gender distribution in Sky Medical and in my own team which I’m proud to say has two female engineers in R&D. According to the Campaign for Science and Engineering, more science A-Levels were awarded to girls than boys in 2019, which is a fantastic transition from when I first started out in the industry.
In your opinion, what will be the key trends in the wearable tech and STEM industry in the next 5 years and where do you see it heading?
Recent years has seen a significant increase in awareness around movements such as #WomenInTech which I believe is incredibly important for younger generations. Within the next five years, I imagine we will see a further positive shift in the number of women in positions of leadership – not just in STEM, but all industries. There are already numerous companies already implementing this change which is leading to a more diverse pool of talented employees and innovative ways of thinking.
With regards to general advancements in technology, I think the coming years will see a huge increase in the amount of smart wearable tech implemented throughout healthcare. Such technology will be able to inform medical professionals about the status of their patients remotely allowing them to triage face to face appointments and tele-medicine where patients are given medical care without them having to leave home. I hope to see the UK continuing to lead the way in wearable healthcare with digital measurement in the coming years.
Who are your 3 inspirational women and / or businesses in wearable tech and / or STEM?
Ophelia Brown – Ophelia has brought a fresh approach to investing in tech startups and companies with her company Blossom Capital. She also founded Ambitions Ladies in Tech in 2016 which is a mentor network helping women in technology startups achieve their career goals.
Anne-Marie Imafidon – Anne-Marie is the CEO and founder of STEMettes. STEMettes is a great organisation which encourages girls aged between five and 22 years old to pursue careers in STEM. Anne-Marie encourages girls and young women to “approach scientific challenges with confidence.”
Dr Jenny Harries – Jenny is the UK’s Deputy Chief Medical Officer and has been playing a huge part in providing clear advice to the UK government and the public about appropriate responses to the COVID-19 pandemic.
References
Website: http://www.skymedtech.com/
This interview was conducted by Marija Butkovic, Digital Marketing and PR strategist, founder and CEO of Women of Wearables. She regularly writes and speaks on topics of wearable tech, fashion tech, IoT, entrepreneurship and diversity. Visit marijabutkovic.co.uk or follow Marija on Twitter @MarijaButkovic.